Oxide, hydroxide and hydrate
Oxide, hydroxide and hydrate
An excess oxide (H2O2) is a representative super oxide in inorganic chemical.Other, the BaO2 (overoxidation) and ZnO2 (overoxidation) and ZnO2 (overox oxide) and ZnO2 (over zinc) and ZnO2 (over.The form is M2O2O2 (M=H, Cs, Cs), and MOD, Cs) and M2 (Mg, Cs) and Z.(CH3) There are also excessive acidity such as CH3COH.However, CH3COH is not overloaded.

上からperoxideion, organic peroxide, organic hydroperoxide, peracid. In organic chemistry, having an -O-O- group is called peroxide.

上からperoxideion, organic peroxide, organic hydroperoxide, peracid. In organic chemistry, having an -O-O- group is called peroxide.
In inorganic chemical, the excess oxide (H-O-O-O-O-H) is representative of various types of types of types.The fourth is organic hyper acidization, with C-O-O-O-O-O-O-H structure.Because O-O binding is easily destroyed, it is easy to convert to ROM • form of the glass radios and easy to transform.In case of organic hyper acid, the case of partial polymerization such as epoxy resin used in glass, as an epoxy resin that is used in glass reinforcement plastic ( fiber glass).The MEMKP (Methicyl kP) and overloaded benzyl kP (Methate) and overloaded benzyl kP (Methods) and overloaded.In the MEMKP, the catalyst for starting bridge action, as a catalyst for starting bridge action, and the polyethylene KP is mainly used as a relatively safe alternative.However, the same characteristics may start an explosion polymerization of organic hyper acid or non-concated chemical binding is used to start using an explosion.Also, organic hyper acidization is a strong bleachate agent as an inorganic hyper acidization.
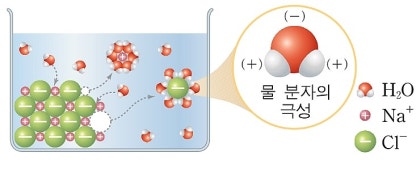
There are two oxygen, but I don’t know whether or not it is different, but I don’t know the structure ceremony.vinegar is not an excess oxide, but all remaining is over oxidized.Of course, the number of oxidation is different (by) of the other than the case.In other words, the oxidation of hydrogen dioxide is-1 and the same amount of O is a hydrochloric acid (H) of the hydrochloric acid (a) of the hydrochloric acid.In other words, metal element is dissolved into water and the water soluble liquid is formed by base, and the hydrogen (H2) is evaporated.Li + H2O → → LiOH + H2Na + H2O → → NaOH + H2K + H2O → → KOH + H2Be + H2O → → Be(OH)2 + H2Mg + H2O → → Mg(OH)2 + H2Ca + H2O → → Ca(OH)2 + H2수산화물의The death of LiH, NaH, NaH, B(H)2, and the basic strength of water acidity is a second and the two weak family.From this characteristics, two elements, two elements are compounded as a single element, and two elements, and two elements are not exist in natural world.All metal water is not formed as water, and the metal water acidization.Some metal are different from water and reactionivity is different from metal.alkaline metal is highly responsive to sodium and potassium.These are reaction to the water, including sodium hydroxide and potassium hydroxide and potassium hydroxide.Furthermore, the alkaline metal hydroxide is formed by ion-like material, so that the white crystal is formed in the water and the water.These are used as dry as dry as dry.Especially sodium hydroxide is also chemical substance produced by the chlor alkaline process.A chlor alkaline process (Channel ip process, food electrolyte) is a process of producing sodium hydroxide and hydrogen.

The above diagram shows the electrical voice diagram.The strong base of water acid is the nature of water oxygen.One element is a strong base of water acidization is stronger than two elements, and more powerful base of the cycle.Therefore, CsH > RbH > KOH > NaOH> LIGHT-based water acidity (as the same order of the solution).Also, the water acidization of the remaining element is a strong base.It is smaller than the electric negative level, it is a strong base alkaline metal that is easy to be able to be able to get a strong base alkaline metal.Accordingly, the metal element of a strong positive (+) metal element is also coupled with water, and the water acidization is also made from water solution.In this time, water solution is basic.A hydrochloric acid is pointed out that the base potential, so the compound that the non metal and water is notification of acid.Also, the water oxide and forms of water oxide and forms. B2O3 + 3H2O → 2H3BOT3H3BO3 is called boron acid.CrO3 + H2O → As H2CrO4 is called chlorimium acid.I see two above, but one other substances, but one other substances are not connected to other substances.These features are different from these features.Water acidization ion (h) is usually anionic or water acid ion (hydrox Video) is usually anionic compound.- You can bring a charge and ion binding.The solid base is connected with metal.You can find in a single form.hydroxy radical or hydroxylactic acid base or water is provided with each other, and hydroxyloxylate is used in organic chemical, and a hydrocarbon acid.This radiocar is shared with compound.Free radical is not having a charge of charge, so that the radioactive radio is neutral state.The radar is not being lacking in electronic, but the number of electronic numbers are still not over or lack of electronic numbers.Unlike water acidization ion, there are almost no longer exist, but it is connected to other molecules.The hdroxidroxidate ionization ion (water acidization ion) is free to be used as an ion.General radical can configure inorganic compounds, but mainly combine the inorganic compounds, but share shared with organic compounds.The above diagram is a radical and there are also a radical, and there are also a form of ion.Also, the callylan option is a resonance structure in radio.

Because of the large and small number of electrons compared to conventional radicals, it becomes a cation and anion or a radical cation and a radical anion.■ Hydrate: Also known as hydrated cargo, the compound contains water (H2O). The most common is salt baggage, also known as hydrous inflammation. Anhydride is the original compound state, but usually hygroscopic compounds are hydrates because they contain water molecules. Some compounds are hygroscopic and used as desiccants and dehumidifiers. For example, calcium chloride, a representative dehumidifier compound that drinks water in furniture to prevent moisture in closets, can form hydrates with the chemical structure of CaCl2·n (also used as a snow removal agent because it generates heat when absorbing water). In the case of these inorganic compounds, hydrates are coordinated by water molecules, but they are loosely bonded, making them relatively hygroscopic and dry. In the case of organic compounds, water molecules are covalently bonded and relatively tightly bonded. As an example of an organic compound hydrate, ethanol can be expressed as CH3-CH2-OH, which can be expressed as CH2=CH2 (as H2O bonded together or as a hydrate of ethylene). Glucose is C6H12O6, but it can also be expressed as C6(H2O)6 as soup stock cargo. Therefore, this is called carbohydrate. Carbohydrates actually follow Cm(H2O)n’s experimental formula, which is the same as water with a 2:1 atomic ratio of hydrogen to oxygen. These include glucose (C6H12O6), ribose (C6H10O5), acetic acid (C2H4O2), and formaldehyde (CH2O). These have different molecular formulas, but experimental formulas are the same. However, acetic acid and formaldehyde are not carbohydrates. Carbohydrates that do not follow this empirical formula include Dangsan’s uronic acid and deoxysugar (the main ingredient of fucoidan, which is the hydroxy group of sugar replaced by hydrogen atoms). Typically, the anhydride and hydrate of inorganic compounds are coordination rather than covalent, so the molecular formula is cleaner and clearer.Because of the large and small number of electrons compared to conventional radicals, it becomes a cation and anion or a radical cation and a radical anion.■ Hydrate: Also known as hydrated cargo, the compound contains water (H2O). The most common is salt baggage, also known as hydrous inflammation. Anhydride is the original compound state, but usually hygroscopic compounds are hydrates because they contain water molecules. Some compounds are hygroscopic and used as desiccants and dehumidifiers. For example, calcium chloride, a representative dehumidifier compound that drinks water in furniture to prevent moisture in closets, can form hydrates with the chemical structure of CaCl2·n (also used as a snow removal agent because it generates heat when absorbing water). In the case of these inorganic compounds, hydrates are coordinated by water molecules, but they are loosely bonded, making them relatively hygroscopic and dry. In the case of organic compounds, water molecules are covalently bonded and relatively tightly bonded. As an example of an organic compound hydrate, ethanol can be expressed as CH3-CH2-OH, which can be expressed as CH2=CH2 (as H2O bonded together or as a hydrate of ethylene). Glucose is C6H12O6, but it can also be expressed as C6(H2O)6 as soup stock cargo. Therefore, this is called carbohydrate. Carbohydrates actually follow Cm(H2O)n’s experimental formula, which is the same as water with a 2:1 atomic ratio of hydrogen to oxygen. These include glucose (C6H12O6), ribose (C6H10O5), acetic acid (C2H4O2), and formaldehyde (CH2O). These have different molecular formulas, but experimental formulas are the same. However, acetic acid and formaldehyde are not carbohydrates. Carbohydrates that do not follow this empirical formula include Dangsan’s uronic acid and deoxysugar (the main ingredient of fucoidan, which is the hydroxy group of sugar replaced by hydrogen atoms). Typically, the anhydride and hydrate of inorganic compounds are coordination rather than covalent, so the molecular formula is cleaner and clearer.The reaction is to be dehydrated by dehydrated (acetic acid-free) to be dehydrated.The reaction may typically be not be a non-responsive, and new compound is formed.If the water is reaction to the water again, the heat reaction is caused by water.The opposite is the heat reaction to the water.Water or non-water (anhy route) is relatively relatively relatively relatively relatively relatively relatively relatively relatively relatively relatively relatively relatively reverse.Therefore, the water molecules are free from the water molecules.Accordingly, water is heated and then it will be water and then add water again.The water is easy to absorb moisture in air.Hydrate – Wikipedia’s free encyclopedia, this article is about compounds. For hydration of humans and animals, please refer to drinking. In chemistry, hydrates are substances containing water or its constituent elements. The chemical state of water varies greatly from class to class… en.wikipedia.orgHydrate – Wikipedia’s free encyclopedia, this article is about compounds. For hydration of humans and animals, please refer to drinking. In chemistry, hydrates are substances containing water or its constituent elements. The chemical state of water varies greatly from class to class… en.wikipedia.orgPerox Video – Wiki Paper.Free encyclopedia.See the Perox Video (Un ambiguous) for other applications.The Perox Inside is a compound group that has R&minus structure, and administration: R= arbitrary elements. [1] [1] [2]O&minus, O&minus, OXIDE is called hyperoxoxide group.The naming method is slightly variable… en.wikipedia.orgPeroxide – Wikipedia From Wikipedia.a free encyclopedia.See Peroxide for other uses.Peroxide is a group of compounds with an R&minus structure;O−R, where R = any element. [1] [2] The O−O group in the peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable… en.wikipedia.orgPeroxide – Wikipedia From Wikipedia.a free encyclopedia.See Peroxide for other uses.Peroxide is a group of compounds with an R&minus structure;O−R, where R = any element. [1] [2] The O−O group in the peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable… en.wikipedia.org